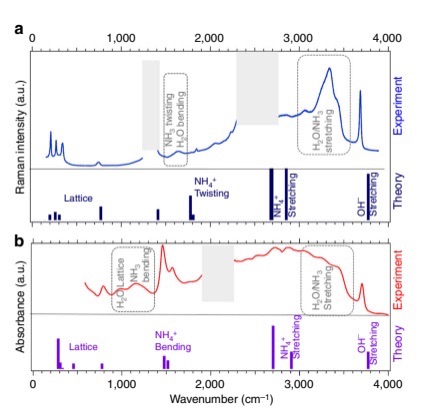
In cooperation with Paris Institute of Mineralogy, Material Physics and Cosmochemistry, associate professor Liu Cailong from IAMP has made new progress in ammonia monohydrate compound. The results were published in Nature Communications on October 20th, 2017. Water and ammonia are considered major components of the interiors of the giant icyplanets and their satellites, which has motivated their exploration under high P–T conditions. Exotic forms of these pure ices have been revealed at extreme (~megabar) pressures, notablysymmetric, ionic, and superionic phases. Here we report on an extensive experimentaland computational study of the high-pressure properties of the ammonia monohydrate compound forming from an equimolar mixture of water and ammonia. Our experiments demonstrate that relatively mild pressure conditions (7.4 GPa at 300 K) are sufficientto transform ammonia monohydrate from a prototypical hydrogen-bonded crystal into aform where the standard molecular forms of water and ammonia coexist with their ionic counterparts, hydroxide (OH−) and ammonium.(NH+4)ions. Using ab initio atomistic simulations, we explain this surprising coexistence of neutral/charged species as resulting from a topological frustration between local homonuclear and long-ranged heteronuclearionisation mechanisms.
The first author of the study is associate professor Liu. The corresponding authors of the paper are professor Frédéric Datchi and Sandra Ninet from Paris Institute of Mineralogy, Material Physics and Cosmochemistry.
Link to the full text of the article: https://www.nature.com/articles/s41467-017-01132-z